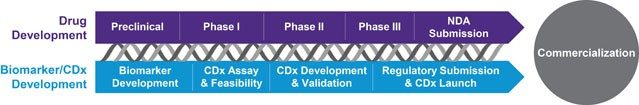
Precision medicine demands patient stratification – identifying target patients who are more likely to benefit from therapy; companion diagnostics also enable reduced development time and Rx development costs, can result in more favorable reimbursement and can illustrate comparative effectiveness.
A new option — called a complementary diagnostic — provides additional information about how a drug might be used, but is distinct from companion diagnostics. We supported the development of the first two FDA-approved complementary diagnostics, and can help you weigh your strategic options.
Your therapeutic needs a companion; you need a resourceful partner.
A companion diagnostic (CDx) can transform the promise of personalized medicine into reality. Backed by our team of medical and scientific subject matter experts across all therapeutic areas, you’ll harmonize your Rx and Dx development objectives. Together, in tandem with your therapeutic approval, we’ll help your CDx reach the market—faster.
The power of choice: in vitro testing or laboratory developed test.
Your best path to CDx commercialization may not involve a traditionally distributed in vitro diagnostic (IVD) kit. Projects involving orphan drugs, compressed timelines or operational complexities may thrive with an FDA-approved, laboratory-based assay. You’ll minimize upfront investment and mitigate potential risks associated with a widely distributed IVD kit. As your partner, we’ll develop and validate your test, lead the regulatory submission and offer immediate global access to get your test to market quickly.
Whether pursuing a laboratory developed test (LDT) or an IVD, you need expert guidance to unite your CDx and therapeutic development and extract the most value from your trials. With more than 25 years of clinical trial experience and 80+ diagnostics delivered to market, we possess the technical arsenal, strategic agility and scientific insights that can help you determine the best approach to commercialization.