Designed Around You
Biomarker Development & Testing
Multidisciplinary and integrated solutions to meet your biomarker strategy goals
Collaboration, scientific design and problem-solving that fits your unique requirements
Deep expertise and insights to drive your preclinical and clinical – including exploratory - biomarker programs – even into commercialization
Innovative, state-of-the-art platforms and global laboratory testing capabilities
Biomarkers are at the epicenter of today’s drug development and the pursuit of personalized medicines, which accounted for more than 40% of FDA approvals in 2018.1 They help define disease biology, drive patient identification and stratification, identify mechanism of action and understand the pharmacodynamic aspects of a drug, among other aspects.
A clear path forward for efficient drug development
To fully realize the benefits of a biomarker strategy, you need early insights, focused expertise and a clear blueprint that includes the most advanced techniques and platforms. It begins with a deep understanding of the role of biomarkers in your program, the proposed application, and which tools will help you achieve your goals.
Selecting the most appropriate regulatory environment for your biomarker program must also be considered. Rapid development, validation and—when needed—transfer of your assay will optimize your timeline and financial impact.
1Personalized Medicine Coalition. Personalized Medicine at FDA: A Progress & Outlook Report, 2018
An approach as unique as your molecule
Led by our Biomarker Solution Center team of PhDs focused on key therapeutic areas, the design for your biomarker program is informed by industry insights and deep specialized knowledge around three key areas:
Role of Biomarker
- Predictive
- Pharmacodynamics
- Monitoring
- Prognostic
- Safety
- Susceptibility/ Risk
- Diagnostic
Intended Use
- Intended Use
- Mechanism of Action Identification
- Surrogate Endpoint
- Patient Identification / Stratification
- Inclusion/ exclusion
- Patient Care
- Companion Diagnostic
Tools
- Next Generation Sequencing
- Flow Cytometry
- Immunohistochemistry
- Immunoassays
- Cell-based Assays
- LC-MS
- Anatomic Pathology
The result is a customized approach that takes into account the entire program, platforms and timely progression toward your milestones.
Our drug development and diagnostic laboratories create an extensive global laboratory testing capabilities, ranging from standard clinical laboratory and safety testing to esoteric and complex biomarker testing, giving you more than 4,500 assays and the ability to develop new assays for your biomarker. Whether your program requires exploratory / fit-for-purpose assays or GLP/GCLP and CAP/CLIA regulated environments, you’ll get efficient placement of your program and cost-effective transfer as your regulatory requirements shift.
Biomarker solutions from bench to commercialization
Seeing the entire drug development process is key to understanding how biomarker data utilization shifts as your program matures – and responding rapidly to keep your timelines intact.
Whether you’re actively planning a CDx registration trial or still seeking to confirm that your biomarker candidate is predictive of response to therapy, a holistic overview of the diagnostic continuum can avoid gaps in regulatory oversight, data integrity and development timelines.
Early engagement with the Labcorp Drug Development Diagnostic Development Services team provides access to industry-leading expertise in drug and diagnostic co-development from concept through commercialization.
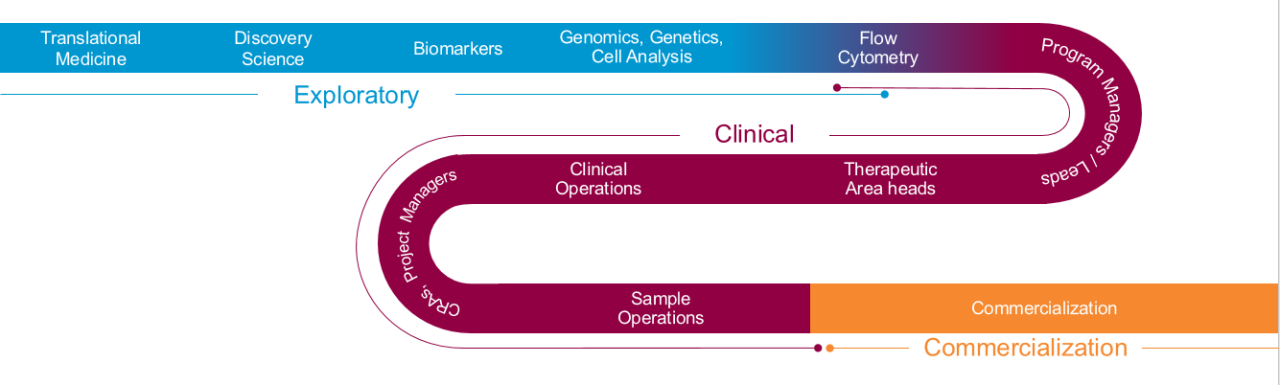
- Exploratory – Start with your target goal in mind – whether exploratory or in support of clinical trials – and team with our translational biomarker experts to establish the right pathway to get you the answers you need, when and how you need them.
- Clinical – Progress into the clinic and speed patient recruitment.
Connect
Let's start the conversation
Contact Us