COVID-19 Vaccine & Antiviral Preclinical Development and Testing
Developing safe and effective vaccines or treatment has never been more urgent than during the COVID-19 pandemic. Extend your coronavirus discovery and preclinical development capabilities for vaccines, antivirals or cytokine treatment by partnering with our experienced team.
Discovery & Anti-Infective
- Immunogenicity
- In vivo models
- BSL-2 facilities
Drug Metabolism & Pharmacokinetics
Safety Testing
Vaccine, Antiviral and Infectious Disease Experience
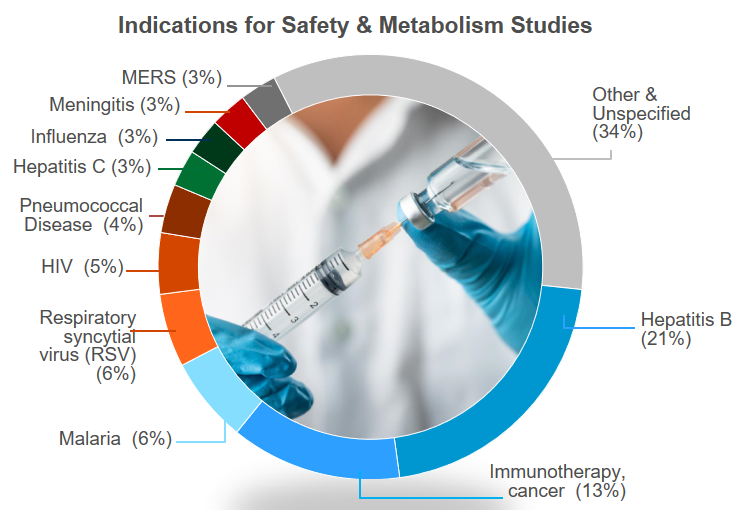
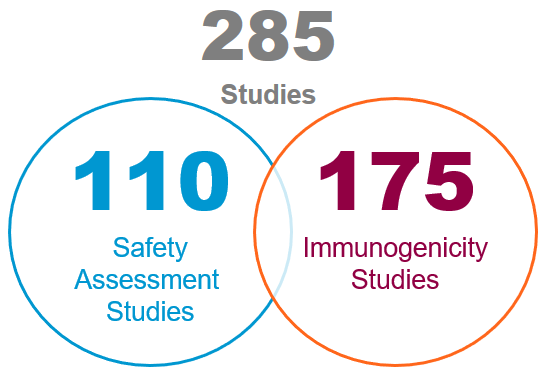
Here's a quick overview of our infectious disease, vaccine and antiviral preclinical testing experience in the past 5 years:
- 8 global nonclinical testing facilities
- > 4,000 global nonclinical staff
COVID-19 Development Acceleration
Choose a programmatic approach for your coronavirus vaccine, antiviral or treatment, Early Phase Development Solutions, to help you reach your IND/CTA, FIH, PH2 and PoC milestones quickly.

Work with drug development leaders who have more than 300 years of combined development experience
Considering Reformulation?
Our team can help you to determine whether you can reformulate an existing treatment and whether specific regulatory steps would already be satisfied.
Continuity. Partnership. Confidence.
Coronavirus Assays
Looking for clinical testing assays?
As a full-service contract research organization, we can help you move a coronavirus vaccine or treatment quickly and efficiently through all phases of development.
Here is a list of COVID-19 clinical testing assays that have undergone expedited validation to be able to enter coronavirus clinical trials:
- Viral load by qPCR
- Viral neutralization
- COVID-19 screening/rapid detection by PCR
- Antibody detection by ELISA
Connect with our COVID-19 Response Team
Contact Us