Author: Sumithra Urs, Ph.D., Scientist, Scientific Development
Date: October 2020
With the development of increasingly complex immunotherapies, biotech/pharma companies are now looking for more relevant and accurate preclinical tumor models to predict precise drug responses.
While preclinical oncology studies aim to mimic human diseases in an animal setting, the greatest challenge researchers face is the translatability of the results. An ideal preclinical tumor model would not only employ the specific cancer subtype but would also simulate the complex microenvironment required for tumorigenesis.
Several recent publications have shown that the site of tumor implant can impact critical parameters such as tumor kinetics, vascularization, architecture, hypoxic environment and sensitivity to anti-tumoral treatments1-4.
The more frequently used subcutaneous (SC) models have the advantage of monitoring tumor measurements by caliper. However, they differ from the visceral tumors in morphology, vessel density, immune cell infiltrates and tumor microenvironment. This is precisely what an orthotopic (OT) tumor model tries to capture, i.e., implantation of tumor cell lines or patient derived xenografts into the organ that matches the tissue type.
In the specific context of immunotherapy, published data has shown that OT tumor models are immunosuppressive with larger abundance of macrophages and can be less sensitive to certain types of immunotherapy than the same model implanted subcutaneously2.
To explore the potential of OT models to provide a more relevant tumor microenvironment while also acting as the primary location of the tumor as compared to their SC counterparts, we have luciferase enabled several syngeneic cell lines to facilitate non-invasive monitoring of tumor progression and metastasis.
Here, in this model spotlight, we present the model development of Pan02-Luc, the murine pancreatic adenocarcinoma cell line. Note: All animal work was approved by the site Institutional Animal Care and Use Committee and was performed in conformance with the Guide for the Care and Use of Laboratory Animals within an AAALAC-accredited program. Humane euthanasia criteria were predetermined on the basis of body weight and defined clinical observations.
Model development of Pan02-Luc: The Murine pancreatic adenocarcinoma cell line
Surgical implantation of Pan02-Luc cells into the head of the pancreas in C57BL/6 mice results in ~100% tumor take and steady tumor growth with a median doubling time of ~6 days as determined by bioluminescence imaging (BLI). Animals had a relatively long median survival (morbidity/mortality) time of 58 days, which allows 5-6 weeks of dosing and efficacy evaluation in this model (Fig. 1). Tumor engraftment and progression did not have any adverse effect on body weights, and the most common clinical observation that lead to euthanasia was abdominal distention (data not shown).
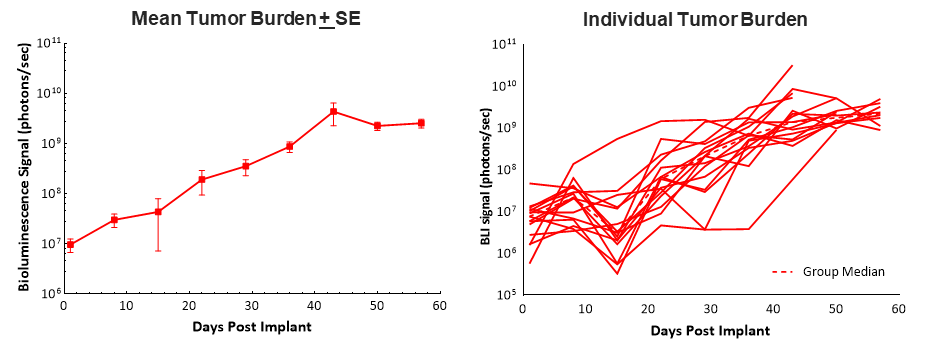
It is well known that pancreatic cancers are considered “cold” tumors and do not respond well to immunotherapies. Therefore, we tested the checkpoint inhibitor anti-mPD-1 alone and in combination with gemcitabine.
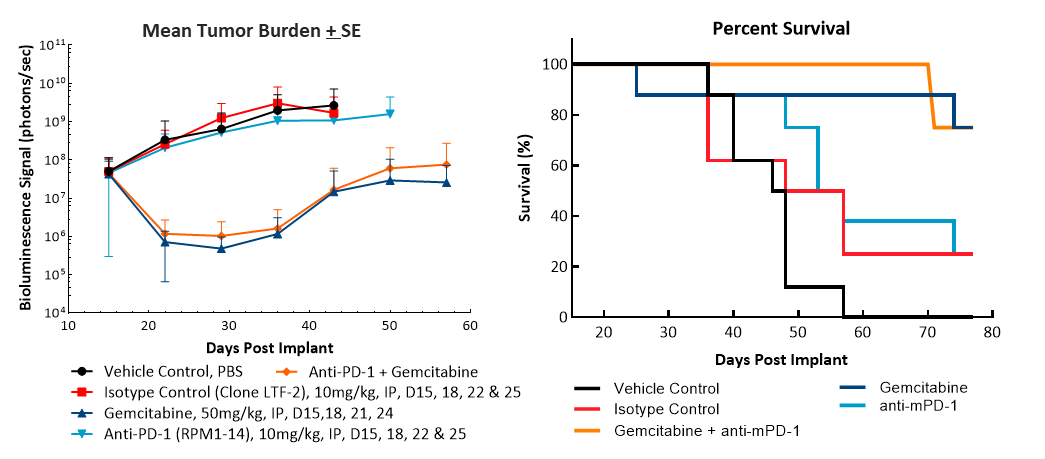
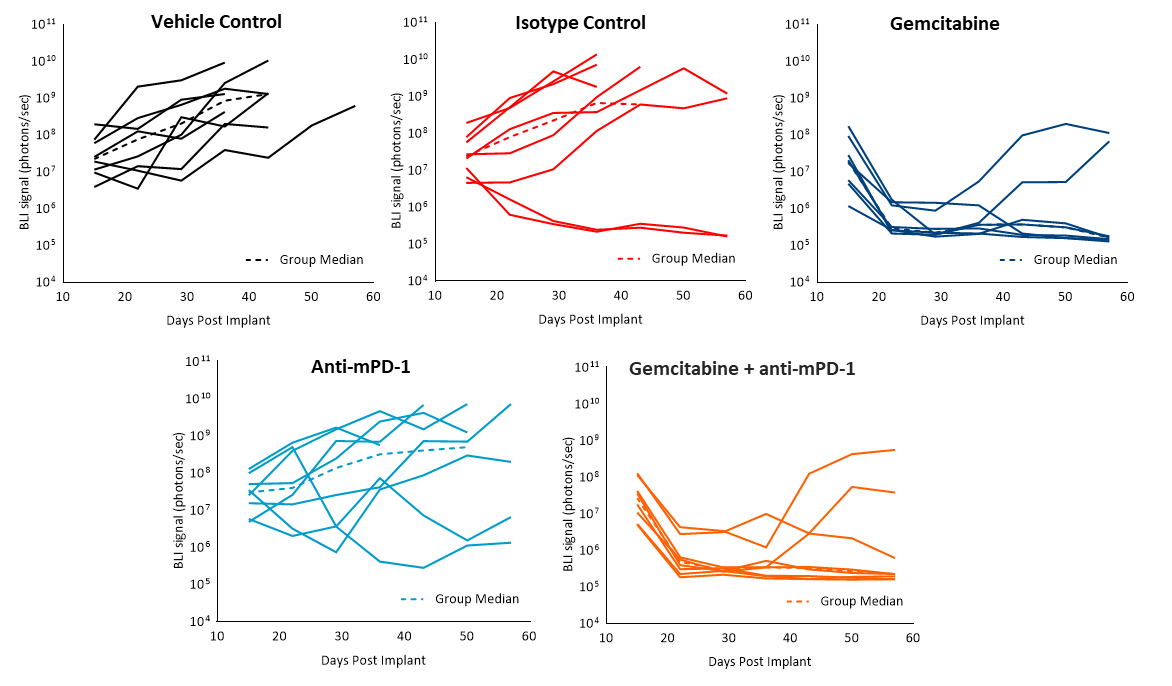
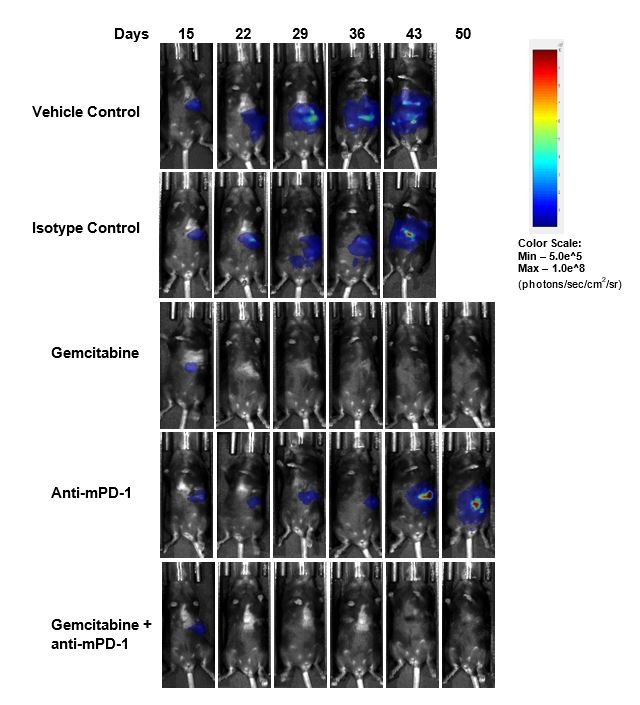
Efficacy evaluation following treatment with anti-mPD-1 (10mg/kg) did not show any meaningful response (Fig. 2). Treatment with the clinically relevant standard of care chemotherapy agent gemcitabine (50mg/kg) resulted in complete tumor regression (CR) in 87% (7/8) of the animals and 62% (5/8) were deemed tumor free survivors (TFS) as determined by BLI. Regrowth was observed in two animals after the end of treatment. The combination of gemcitabine with anti-mPD-1 did not result in any additional TFS over single agent gemcitabine. Representative bioluminescence images (Fig. 2C) and histology images (Fig. 2D) from tumors taken at the end of the study confirm tumor regression in the gemcitabine treated groups.
Fig. 2. Pan02-Luc response to anti-mPD-1 and gemcitabine treatments
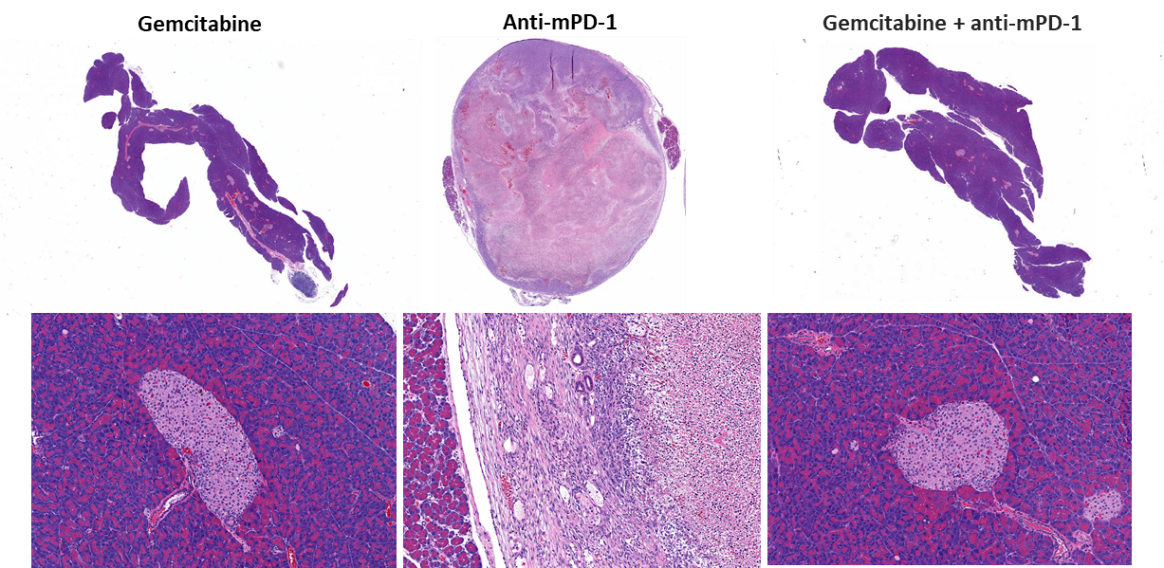
We have previously reported on the response of the subcutaneous Pan02 model to immunotherapy (See this previous model spotlight). Thus, in a comparison to the OT data shown here with the Luc enabled Pan02 cell line, we found that the response to anti-mPD-1 in the OT model was similar to the SC parental Pan02 model. Response to gemcitabine, given at the same dose concentration, was significantly higher in the OT model than in the SC model where animals exhibited tumor growth delay and 80% PR (partial regression), 20% CR, and no TFS (Table 1).
Table 1. Comparison of SC and OT Pan02 tumor response to gemcitabine treatment
Immune cell infiltration into the OT tumors was evaluated by flow cytometry which confirmed the poor lymphoid cell infiltration characteristic of a non-immunogenic or “cold” tumor model (Fig. 3A-B). While CD8+ and CD4+ helper T cells constituted only 1.6% and 1% of the CD45+ population, respectively, the immunosuppressive myeloid cell populations were present in abundance with monocytic-myeloid derived suppressor cells (M-MDSCs) and M1 and M2 tumor associated macrophages (TAMs) being the dominant cell subpopulations. Comparison of the immune profiles of the OT and the SC models showed regulatory T cells (Tregs), Natural Killer cells (NK) and Natural Killer T cells (NKT) cells being significantly reduced in OT tumors. The myeloid population, in general, was larger in OT tumors constituting up to 82% of CD45+ cells, whereas, it was 68% in the SC model. Granulocytic-MDSCs and M2-TAMs were significantly higher in the OT tumors while the dendritic cells were lower and indicative of a more anti-inflammatory immunosuppressive phenotype.
Fig. 3. Flow Cytometry Analysis of SC and OT Tumors
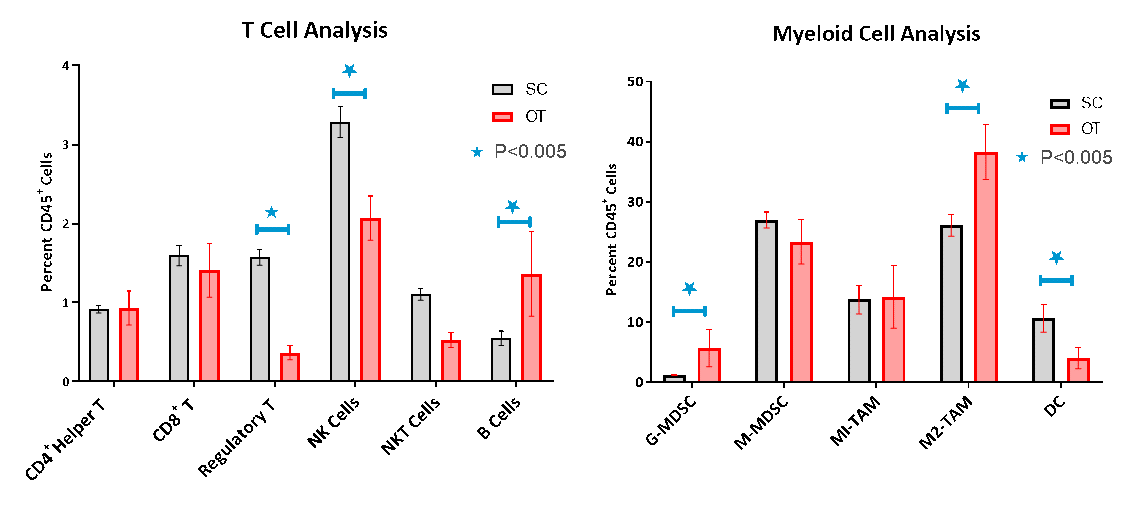
In our hands, these subtle differences between the SC and the OT models may provide insight on the intensity of response to the treatments we have investigated. These differences have been reported to be critical clinically since they influence the predictability of response and prognosis in pancreatic cancer patients5-7.
Orthotopic tumor implantation offers modelling of cancer progression and drug response in a manner that better simulates clinical scenarios. Since each organ and its microenvironment is unique and cannot be replicated by subcutaneous implantation, it is important to use the orthotopic model when available.
At preclinical oncology, we offer several luciferase-enabled models for this purpose.
Please contact us if you are interested in discussing this model further.
References
1. Erstad DJ, et al., 2018. Orthotopic and heterotopic murine models of pancreatic cancer and their different responses to FOLFIRINOX chemotherapy. Dis Model Mech., Jul 1; 11(7): dmm034793
2. Devaud C, et al., 2014. Tissues in different anatomical sites can sculpt and vary the tumor microenvironment to affect responses to therapy. Mol Ther., Jan 22(1):18-27
3. Guerin MV, Finisguerra V, Van den Eynde BJ, Bercovici N and Trautmann A, 2020. Preclinical murine tumor models: a structural and functional perspective. eLife., 2020;9:e50740
4. Qui W and Su GH. 2013. Development of orthotopic pancreatic tumor mouse models. Methods Mol Biol., 980:215-223
5. Kurahara H, et al., 2011. Significance of M2-polarized tumor-associated macrophage in pancreatic cancer. J Surg Res., May 15; 167(2):e211-e219
6. Parente P, et al., 2018. Crosstalk between the tumor microenvironment and immune system in pancreatic ductal adenocarcinoma: Potential targets for new therapeutic approaches. Gastroenterology Res and Practice. 2018:7530619
7. Lankadasari M, et al., 2019. TAMing pancreatic cancer: combat with a double-edged sword. Mol Cancer 18:48
Connect
Let's start a conversation
Contact Us