Author: Sumithra Urs, PhD | Scientist, Scientific Development
Date: June 2018
In this spotlight, we present data from our initial growth and efficacy studies with the E0771 model. The data presented here provides an overview of the E0771 cell line’s response to immuno-oncology agents and radiation therapy to enable design of rational combination studies.
Triple negative breast cancer (TNBC) is a complex and aggressive subtype of breast cancer lacking estrogen receptor, progesterone receptor, and HER2 amplifications; making it difficult to target therapeutically. Consequently, there’s a constant demand for better treatment options for TNBC. To help address the need for TNBC models, we highlighted the EMT6 model last month and herein we put forth the E0771 model, another TNBC syngeneic model for use in preclinical immuno-oncology. The E0771 cell line is a spontaneously developing medullary breast adenocarcinoma from C57BL/6 mice.[1] Parental E0771 is poorly metastatic when compared to 4T1[2] and has homozygous mutations in the Trp53 and KRAS genes.[3]
In this spotlight, we present data from our initial growth and efficacy studies with the E0771 model. The data presented here provides an overview of the E0771 cell line’s response to immuno-oncology agents and radiation therapy to enable design of rational combination studies.
Tumor growth kinetics for the E0771 model is shown in Figure 1. The median doubling time is ~5-6 days with a steady increase in tumor volume with no apparent tumor related body weight loss. The growth rate allows for a three-week therapeutic window to evaluate anti-tumor responses. The growth parameters of untreated tumors and isotype control (Rat IgG2b) treated tumors are similar (Fig. 1).
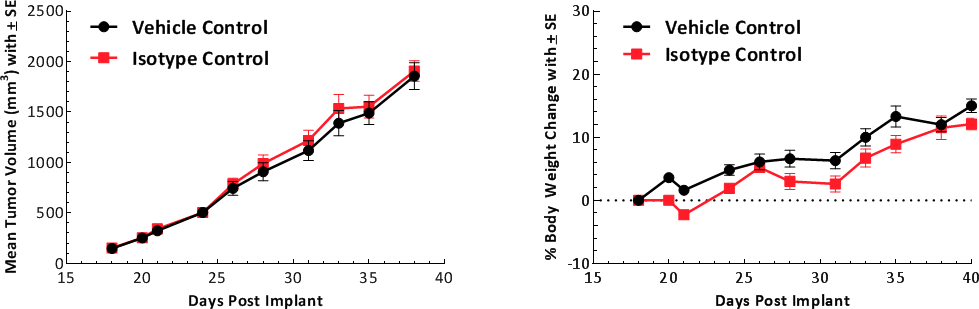
Fig. 1: Growth kinetics of subcutaneous E0771 tumors. Graph showing vehicle control compared to isotype control.
Composition of Tumor Infiltrating Immune Cells
The baseline tumor immune composition was analyzed by flow cytometry and is shown in Fig. 2. The immune profile for E0771 was established from tumors ~750mm3 in volume. The tumors have a high CD45+ cell (70%) infiltration of which the T-cell population (CD8+ T cells and CD4+ T helper cells) are well represented (6% each). The Treg population was consistently lower than the CD4+ T helper cell or CD8+ T cell population. Within the myeloid population, E0771 tumors are unique among our characterized breast cancer lines in having an abundance of M-MDSCs and a near absence of G-MDSCs. M1 macrophages were consistently lower than M2 macrophages in the tumors analyzed. This composition is suggestive of an immunosuppressive microenvironment.
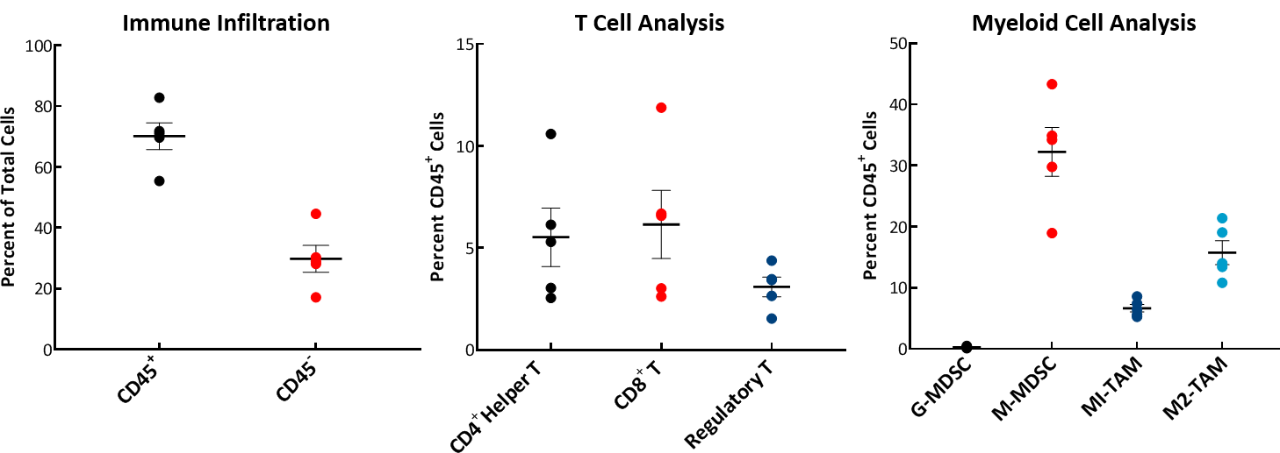
Fig. 2: Tumor immune profile of E0771 showing immune cell infiltration.
Responses to Treatments
Response to immunomodulatory agents was evaluated on mice bearing E0771 tumors treated with checkpoint blockade antibodies (anti-mPD-1, anti-mPD-L1, anti-mCTLA-4, and anti-mLAG3), costimulatory antibodies (anti-mGITR, anti-mOX40, and anti-mCD137) and focal radiation. We tested anti-tumor response to checkpoint inhibitors and costimulatory antibodies in which dosing was initiated prior to palpable tumor formation (Fig. 3). Anti-mPD-1 treatment resulted in complete elimination of the tumor, while anti-mPD-L1 and anti-mLAG3 had significant responses with tumor growth delay (TGD) of 12.7 and 7.6 days respectively (Fig. 3A). Likewise, treatment with costimulatory antibodies anti-mGITR, anti-mCD137, and anti-mOX40 elicited substantial responses with over 50% tumor free survivors (TFSs) and TGD >27 days (Fig. 3B).
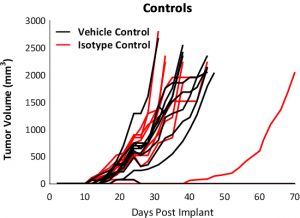
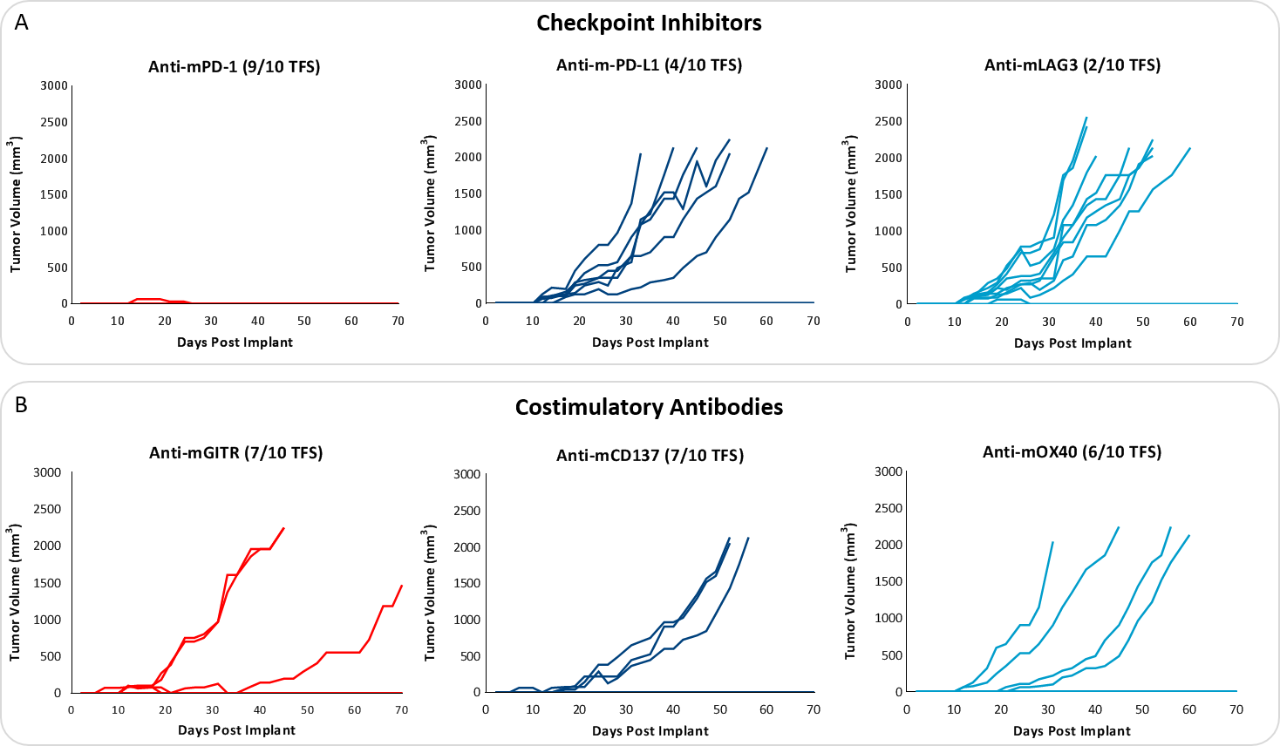
While these results clearly indicate responsiveness of E0771 to immunotherapy (Fig. 5), it simultaneously emphasizes the importance of factors like tumor volume upon initiation of treatment and treatment regimen on outcome. The immunosuppressive microenvironment could help explain the limited response with most of the immunotherapy regimens tested. Focal beam radiation can be a useful treatment modality to modify an immunosuppressive microenvironment. Thus, focal radiation doses 5, 10, and 20Gy were tested and found to be well tolerated with TGD of 5.1, 6.0, and 10.6 days, respectively (Fig. 6). Based on the baseline radiation dose response data, 10Gy focal radiation could be recommended for combination therapies.
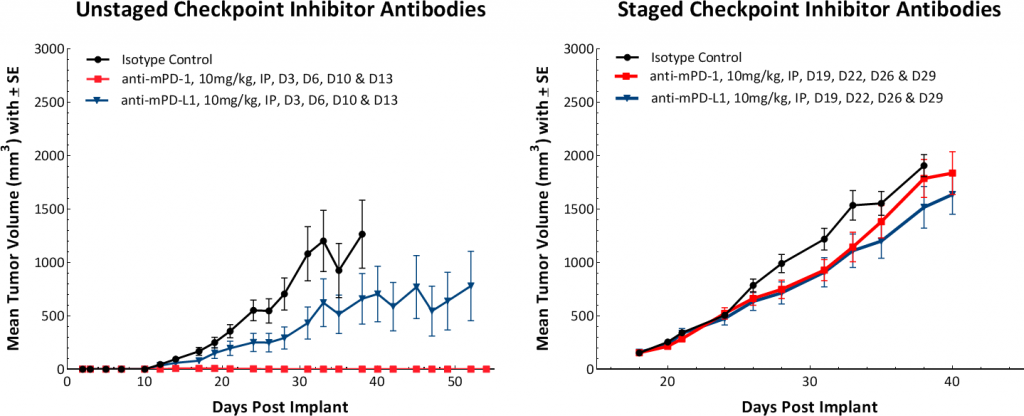
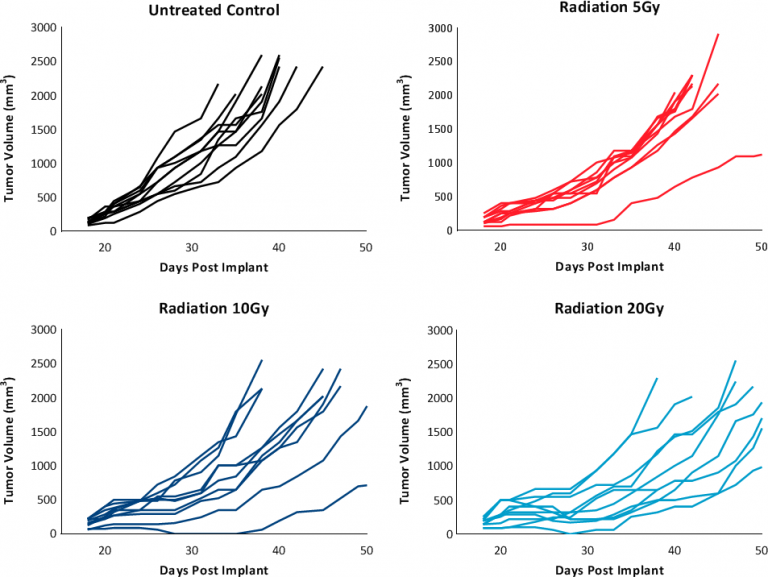
Fig. 6: Response to focal radiation therapy.
E0771 – A Powerful Preclinical Immuno-Oncology Breast Cancer Model
TNBC’s in general are resistant to monotherapy with immunotherapy and are regarded as immunologically “cold” cancers due to their minimal tumor CD8+ T cell infiltrate and low immunogenicity due to a low mutational burden. Given the moderately favorable immune cell infiltration and response to immunotherapy, the E0771 syngeneic breast carcinoma model offers significant potential as a preclinical immuno-oncology model. Our data provide an overview of the effects of each monotherapy and informs design of combination strategies. Treatment start time and tumor volume play a critical role in determining the dynamics of the outcome and should be of strong consideration in study design using the E0771 model.
Please contact us to speak with our scientists about how E0771, or one of our other syngeneic models, can be used for your next immuno-oncology study.
References
[2]Johnston CN, Smith YE, Cao Y et al., (2015). Functional and molecular characterization of E0771.LMB tumors, a new C57BL/6-mouse-derived model of spontaneously metastatic mammary cancer. Dis. Model Mech, Mar; 8(3):237-51.
Connect
Let's start a conversation
Contact Us