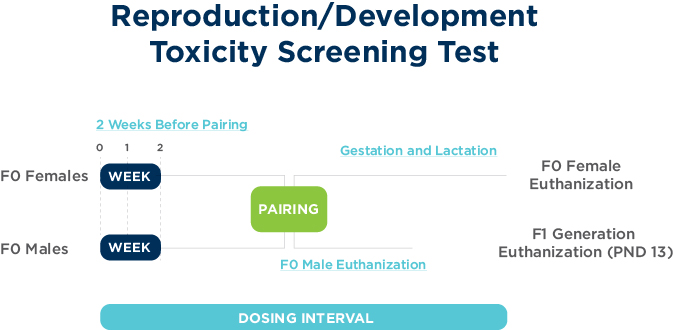
OECD 421: Reproduction/developmental toxicity screening test
The objectives of the Reproduction/Developmental Toxicity Screening Study are to examine chemical exposure on male and female reproductive performance. This study design has been updated with endocrine disruptor endpoints; in particular, the measure of anogenital distance and male nipple retention in pups and thyroid examination.
Other endpoints for this study design include general performance, estrous cycles, mating performance, littering, offspring survival development and growth to mid-lactation, organ weights and histopathology of reproductive organs and the thyroid.
This study design is commonly conducted in rodents via dietary or oral gavage administration.