Cloudman S91 – a responsive syngeneic melanoma model
Author: Sumithra Urs, PhD, Scientist, Scientific Development
Date: April 2021
Of all the skin cancer types, melanoma is the most serious form of skin cancer and while accounting for only about 1% of skin cancer cases, it is associated with the highest mortality1. Melanoma begins in melanocytes of the skin's epidermis, but once it becomes invasive into the skin or other parts of the body, it is more difficult to treat and can be fatal.
The American Cancer Society's estimates for melanoma in the United States for 2021 are about 106,110 new melanomas to be diagnosed (about 62,260 in men and 43,850 in women) and about 7,180 people are expected to die of melanoma (about 4,600 men and 2,580 women). Fortunately, melanoma is curable, when detected and treated early, with a 98 percent estimated five-year survival rate for U.S. patients2.
In the preclinical setting, the most commonly used syngeneic tumor model is the B16 melanoma. As previously described, the B16-F10 model is highly refractory to most immunomodulators and moderately responsive to focal radiation. We now have characterized the Cloudman S91 syngeneic tumor model, described here, as an additional melanoma model for preclinical oncology studies. In the examples shown below, animal care and use was conducted according to animal welfare regulations in an AAALAC-accredited facility with IACUC protocol review and approval.
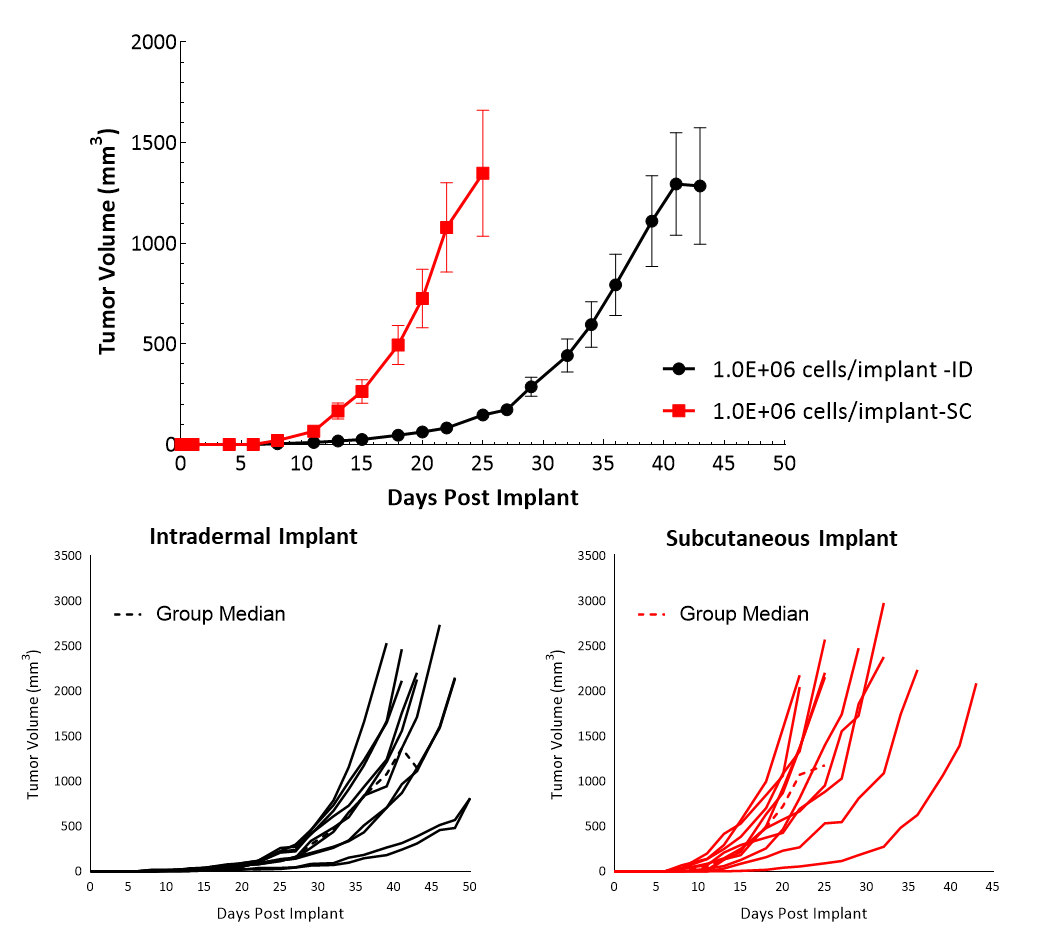
Cloudman S91, also called Clone M-3 or S91, is a melanocyte-derived epithelial mouse melanoma cell line from a male DBA/2 mouse. Clone M-3, a melanin-producing cell line, was adapted to cell culture3 from a Cloudman S91 melanoma in a (C X DBA) F1 male mouse obtained from the Jackson Memorial Laboratory, Bar Harbor, Maine. In vitro, Cloudman S91 cells grow as an adherent population taking on an epithelial morphology. In vivo, intradermal implant of these cells in DBA/2J mice results in delayed growth compared to subcutaneous implant. Subcutaneous tumor cells implant in the high axilla with an initial inoculum of 1.0E+06 cells show efficient growth kinetics with a mean doubling time of approximately four days (Figure 1). Animals stay on study for about 20-25 days before they reach euthanasia criteria of excessive tumor burden.
Cloudman S91 tumor immune profile
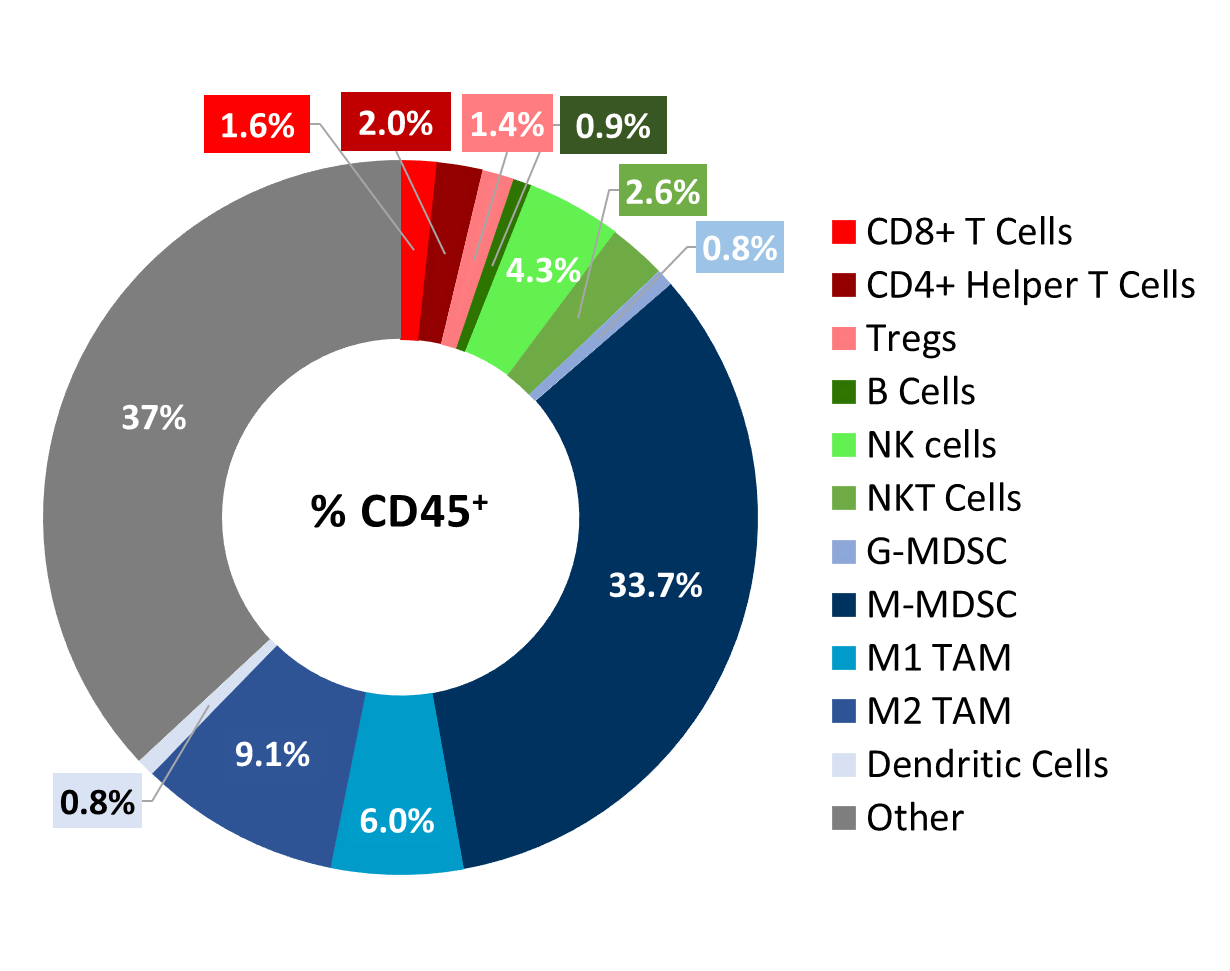
Baseline immune profiling of Cloudman S91 tumor cell infiltrates was measured with flow cytometry on six untreated tumors (300-500mm3) and analyzed using the CompLeukocyteTM package. The cell infiltration in the tumor microenvironment, represented as percent of live CD45+ cells, showed a distinct immune cell population dominated mostly by undefined CD11b+ myeloid cells and a large proportion of M-MDSCs (34%) (Figure 2). M2 TAMs (9%) and M1 TAMs (6.0%) were represented in relatively proportionate extents while G-MDSCs (0.8%) and dendritic cell (0.8%) populations were minimally represented. The lymphoid population was mostly comprised of NK (4.3%) and NKT (2.6%) cells with minimum T cell infiltration.
Cloudman S91 response to therapy Immune Modulators
Immunotherapy with checkpoint inhibitors is currently used to treat advanced melanomas. To understand the efficacy of checkpoint inhibitors against Cloudman S91, animals harboring Cloudman S91 subcutaneous tumors were treated with antibodies against PD-1, PD-L1, and CTLA-4. Treatment of Cloudman S91 tumors (~100mm3) with the checkpoint inhibitors anti-mPD-1 and anti-mPD-L1 resulted in increases in time to progression (%ITP) of 116 and 41, respectively, (Figure 3). Treatment with anti-mCTLA-4, on the other hand, resulted in over 283% ITP and 20% tumor free survivors (TFS). Although the high myeloid content of Cloudman S91 tumors suggests an immune-suppressive tumor microenvironment, single agent immune modulators were able to elicit an anti-tumor effect, suggestive of an immunologically responsive tumor model, unlike the B16-F10 model.

Radiation
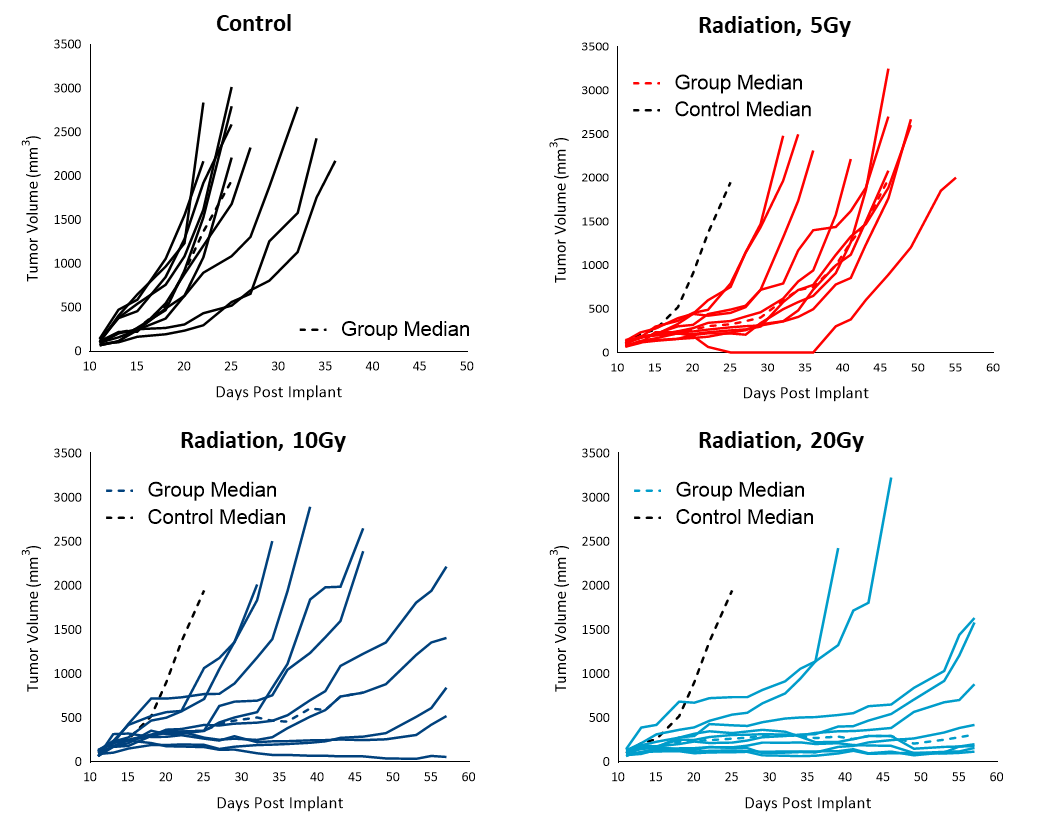
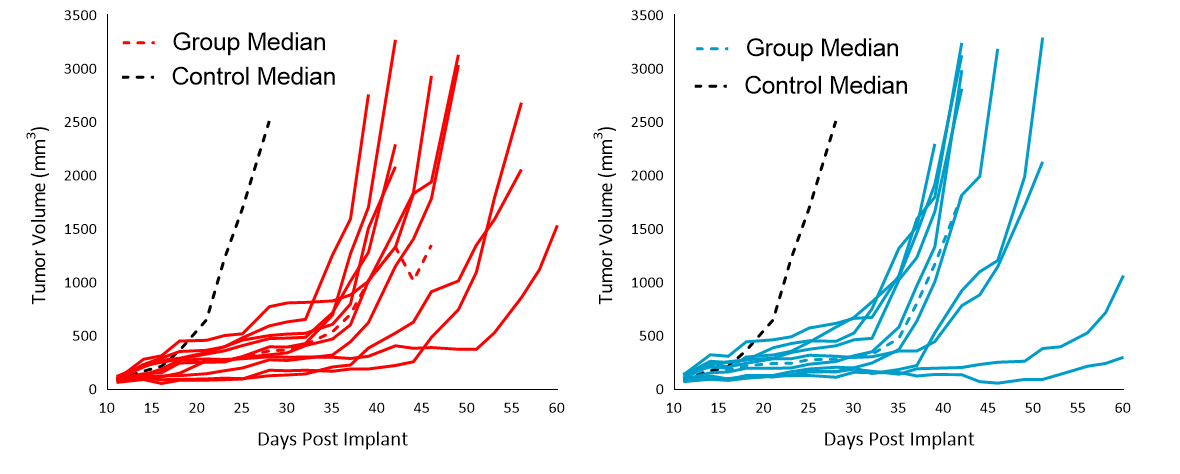
Radiation therapy (RT) is used in cases where patients are poor candidates for surgery or refuse surgical treatment. We evaluated the sensitivity of subcutaneous Cloudman S91 tumors to single dose focal radiation delivered by Small Animal Radiation Research Platform (SARRP) from Xstrahl. Single radiation treatments (2.5, 5, 10 and 20 Gy) showed anti-tumor activity in all animals, resulting in a dose-dependent response (Figure 4). The treatment doses resulted in 50 to over 283% ITP among the radiation-treated groups. Although all the higher doses of treatments resulted in regression, only 20Gy resulted in 10% TFS. A lower dose of radiation can be useful in evaluating combination treatment approaches to mimic clinical treatment regimens that include RT.
Chemotherapy
As shown in Figure 5, evaluation of the chemotherapy agents cisplatin (5mg/kg) and paclitaxel (20mg/kg) against Cloudman S91 showed sensitivity compared to control, resulting in 143 and 121% ITP, but no TFS.
Treatment options for melanoma patients using combination approaches with immune-modulatory agents and chemotherapy or radiation therapy are potential game-changing avenues to improving patient response. In addition, combination treatments may also contribute to altering the immunologically cold nature of the tumor towards a more receptive/responsive tumor microenvironment that would become more amenable to therapy. To discuss how the Cloudman S91 model would be useful in your next immunotherapy study, contact our scientists.
References
1. https://www.cancer.org/cancer/melanoma-skin-cancer/about/key-statistics
2. https://www.skincancer.org/skin-cancer-information/melanoma/melanoma-warning-signs-and-images/
3. Yasamura Y, et al. Establishment of four functional, clonal strains of animal cells in culture. Science 154: 1186-1189, 1966. PubMed: 4288399
Connect
Let's start a conversation
Contact Us